血液动力学专栏Assessment of cerebral aneurysms
风流知音【血液动力学专栏】Assessment of cerebral aneurysms and other vascular diseases using a fully circulative vessel network simulation tool CFDJC(2018)1010
Assessment of cerebral aneurysms and other vascular diseases using a fully circulative vessel network simulation tool
Abstract
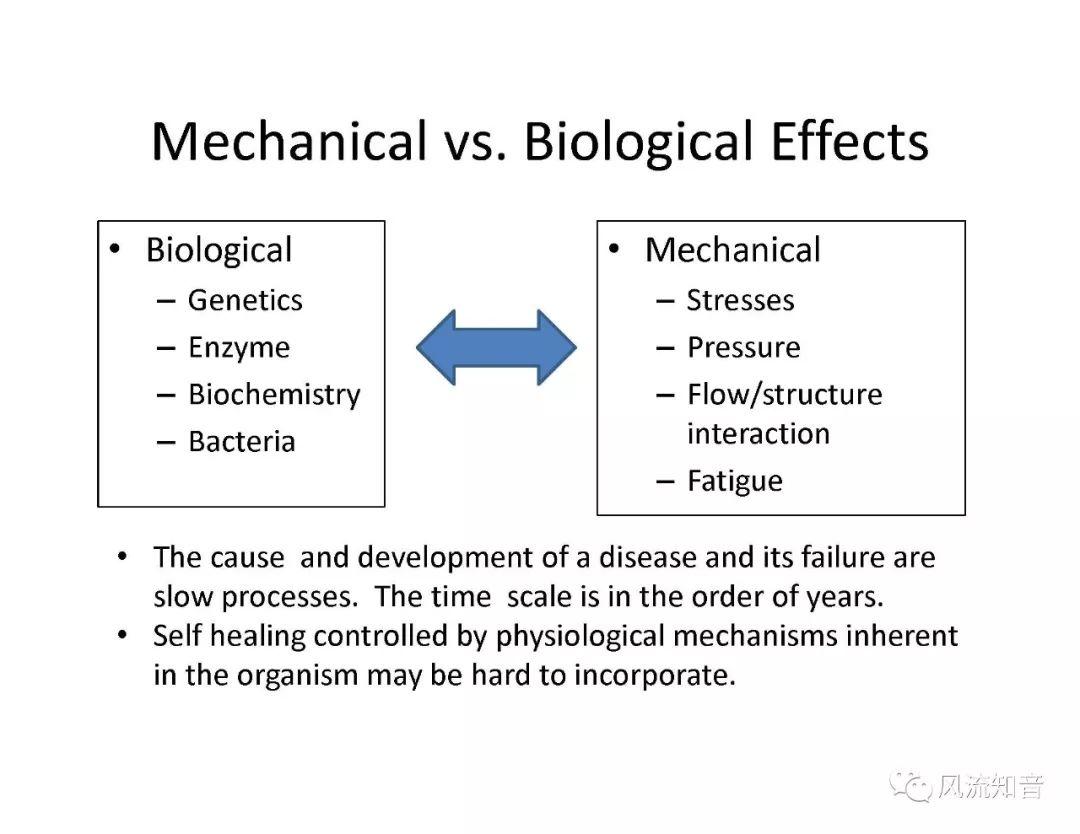
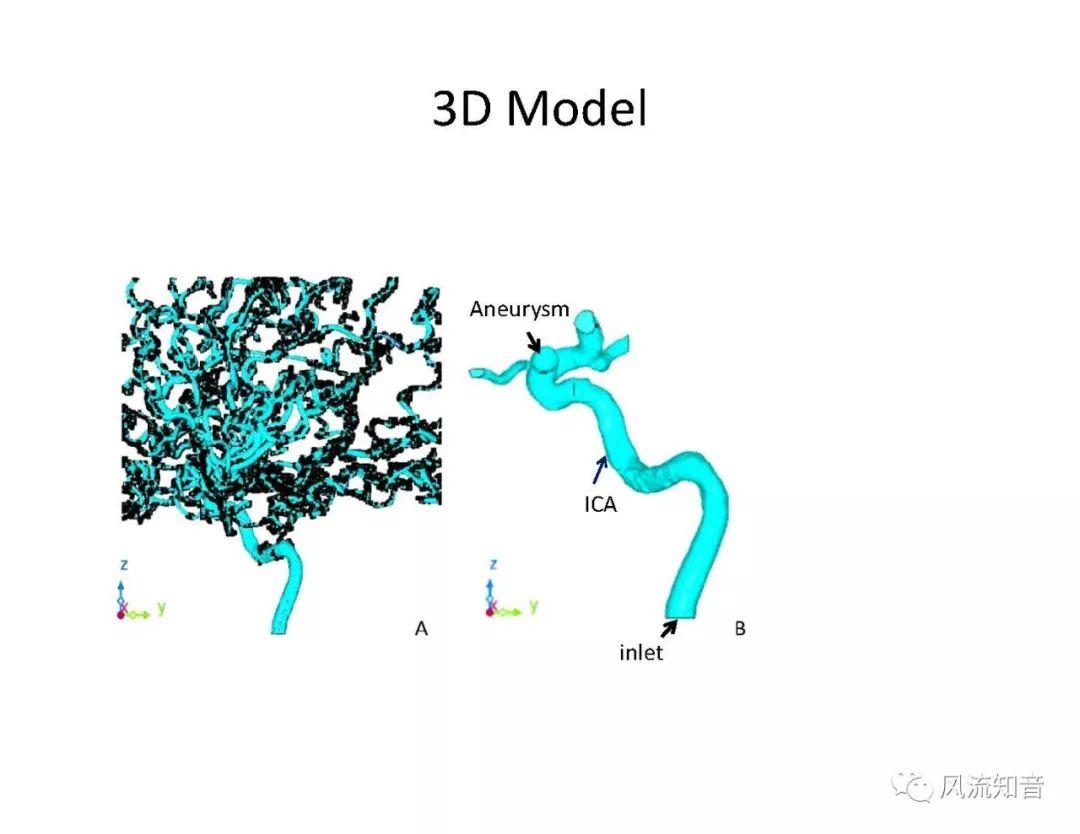
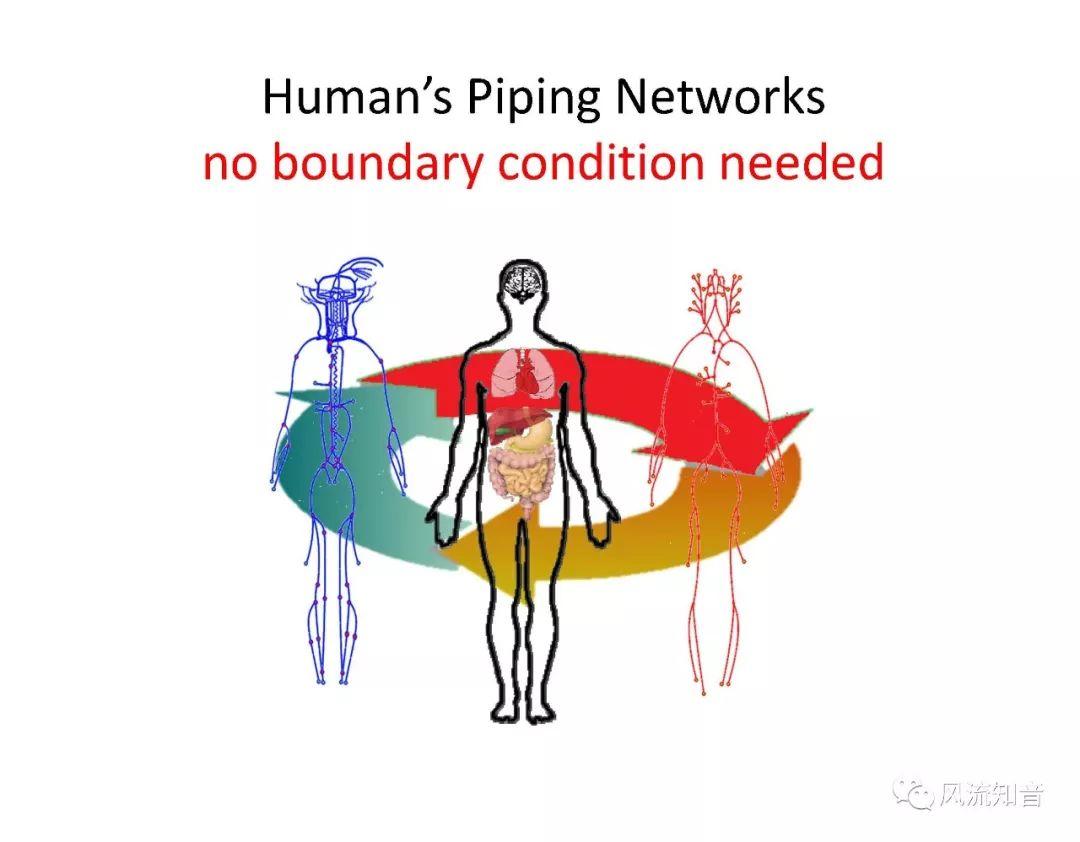
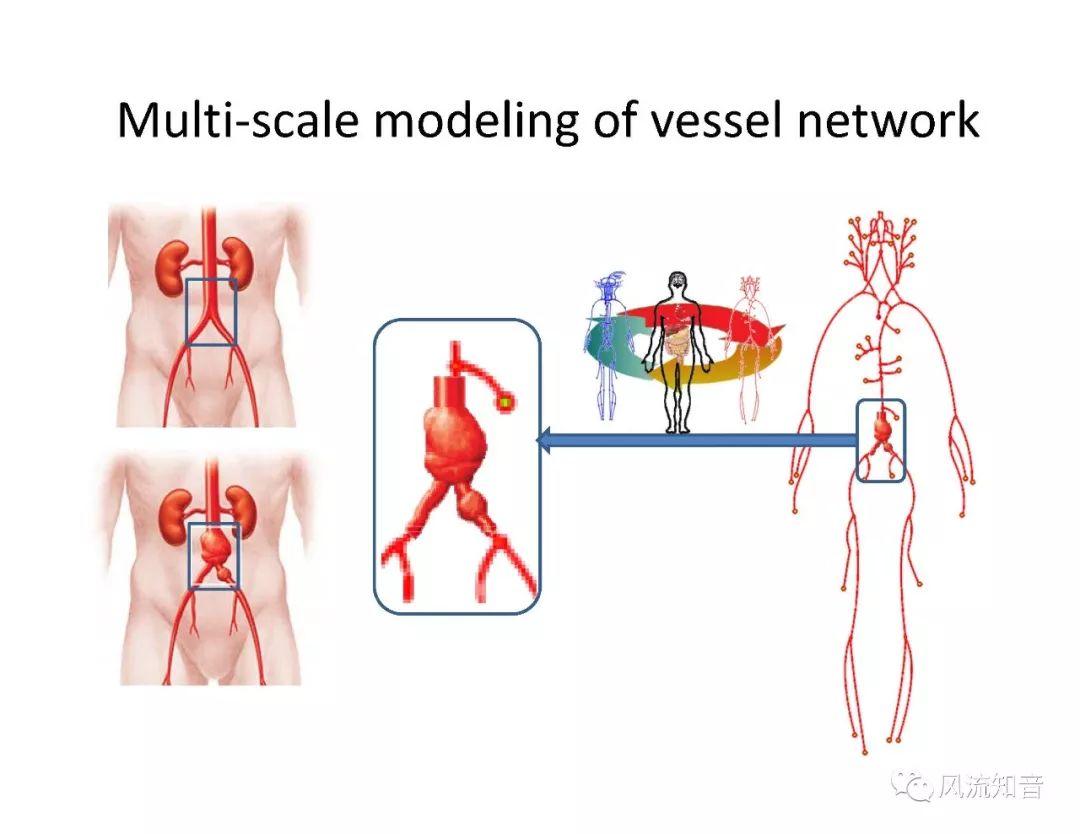
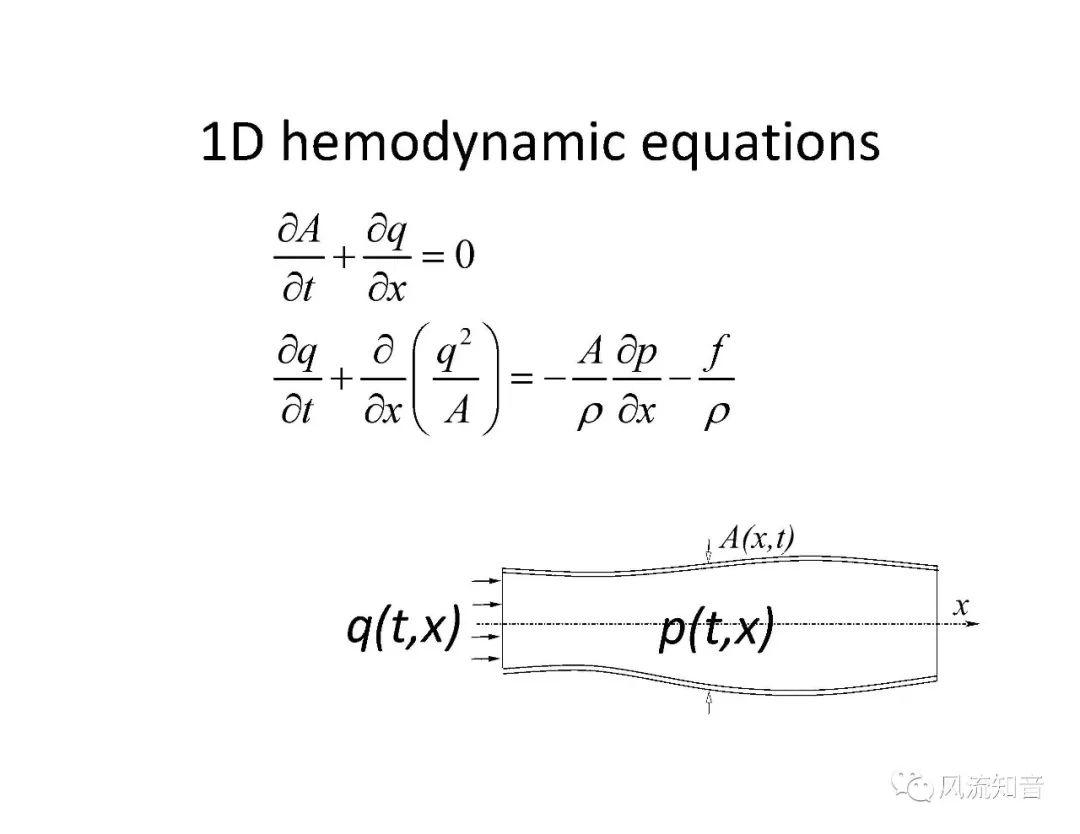
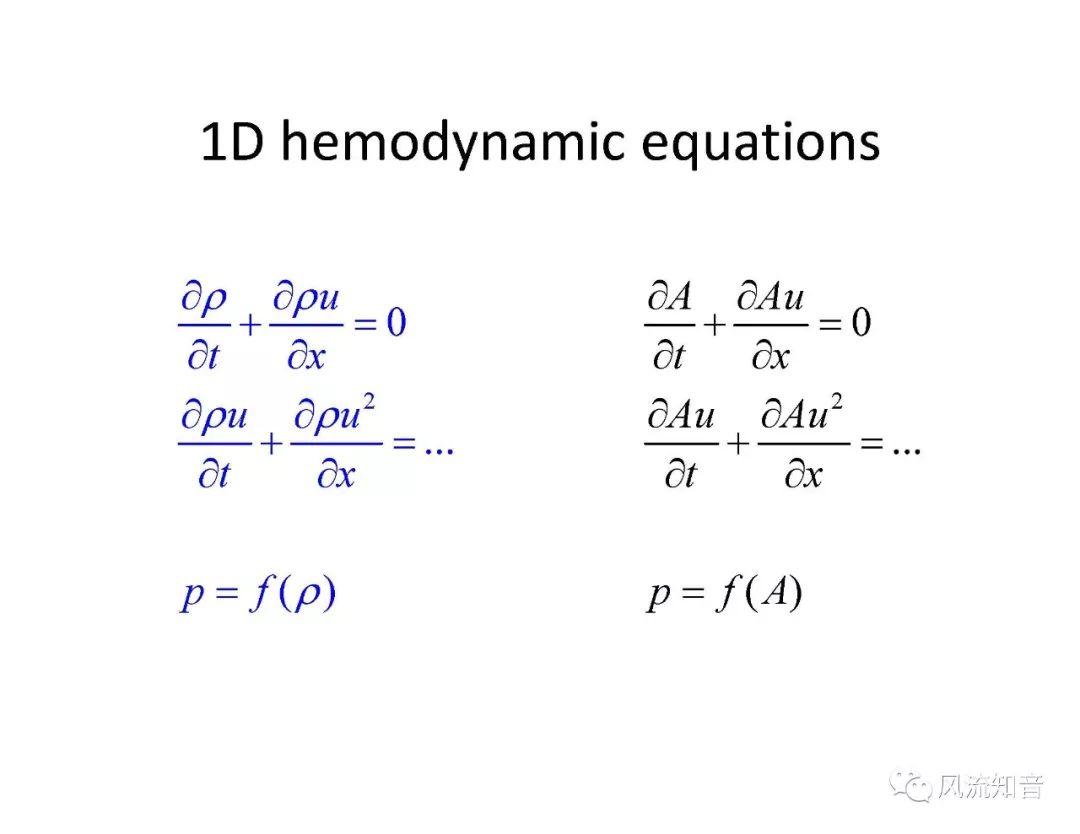
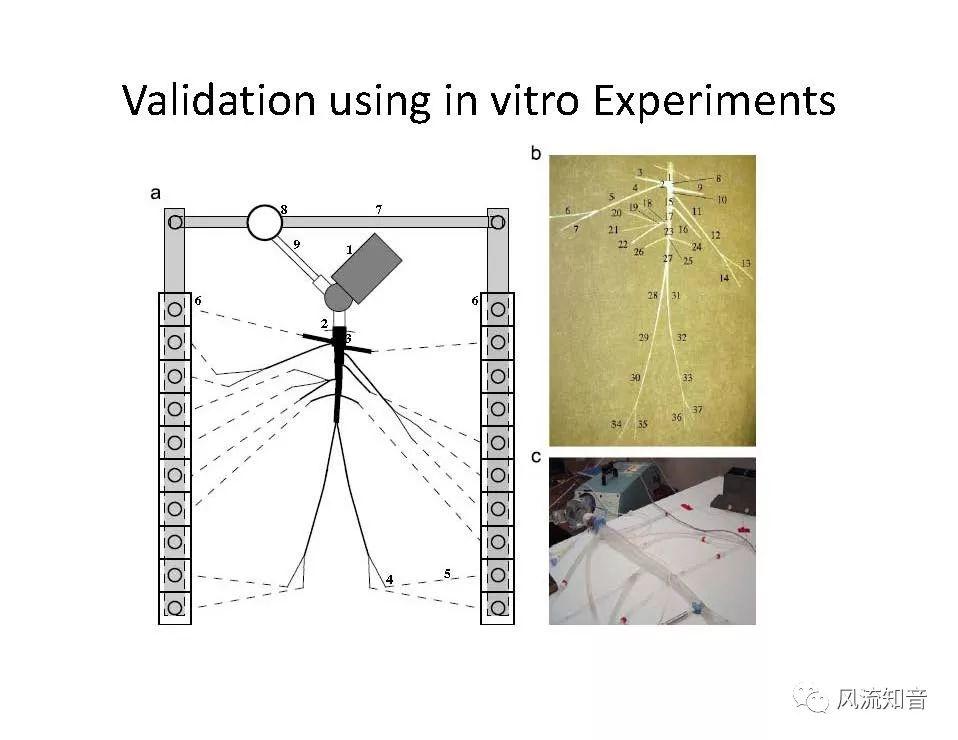
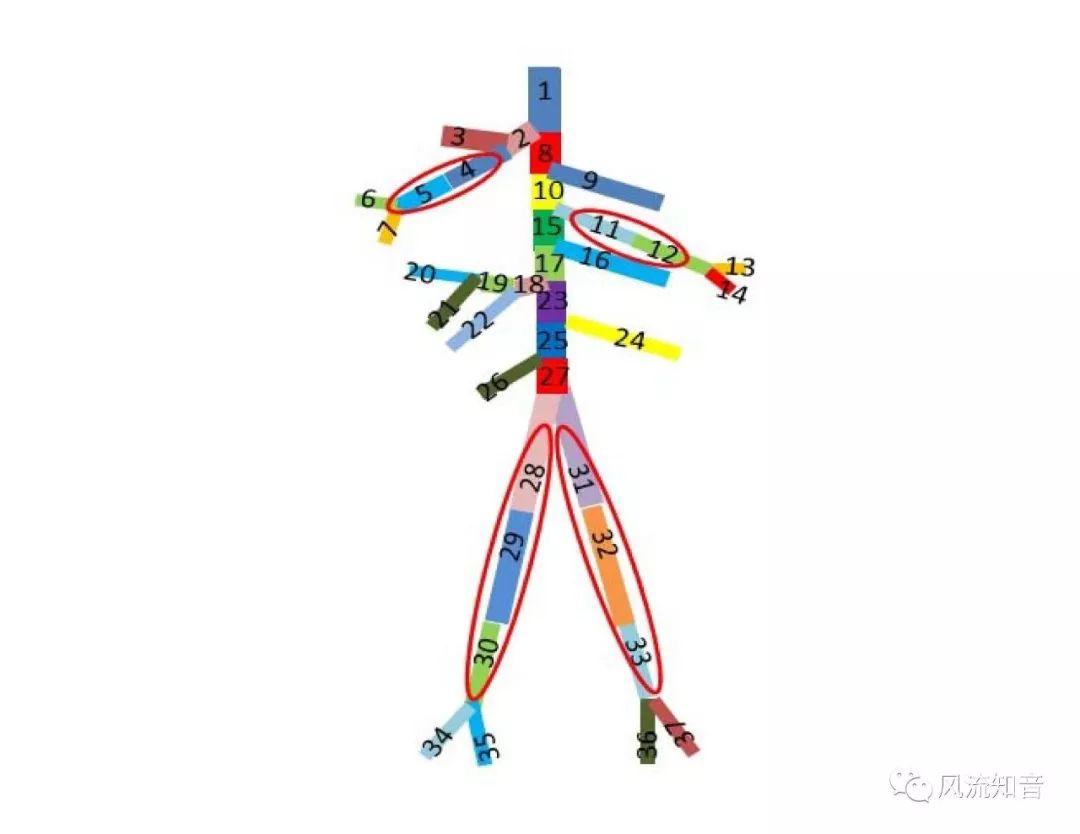
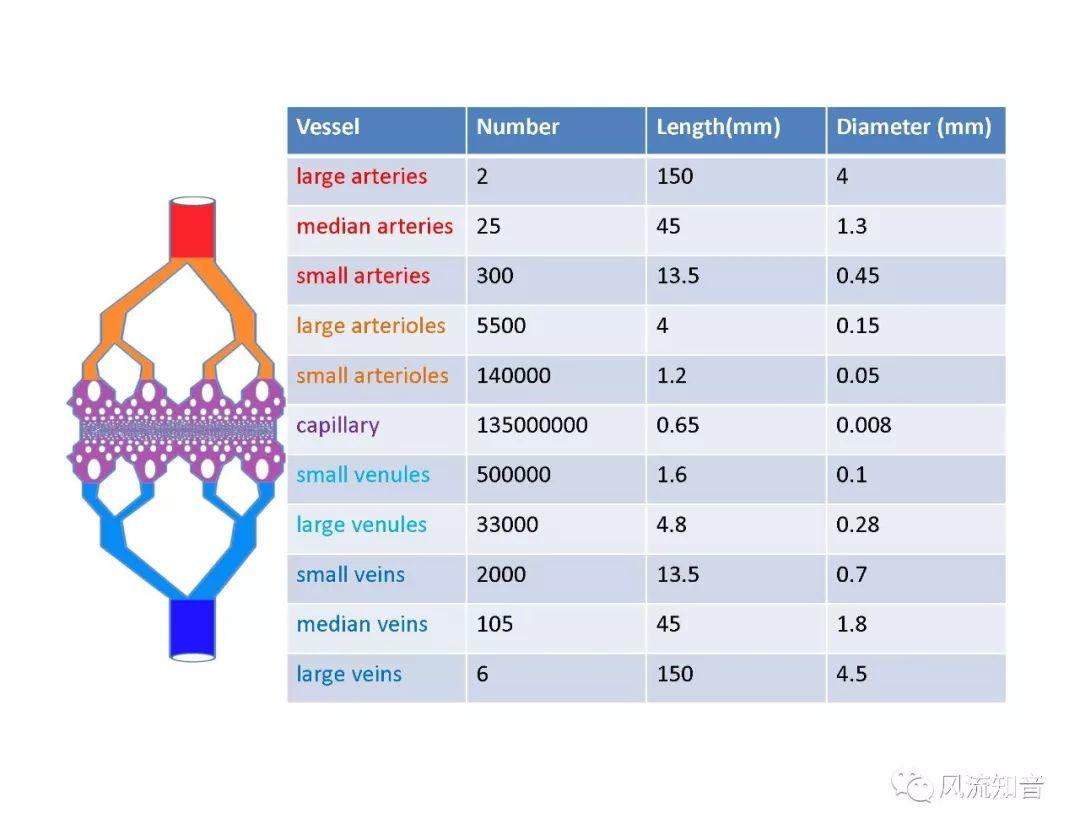
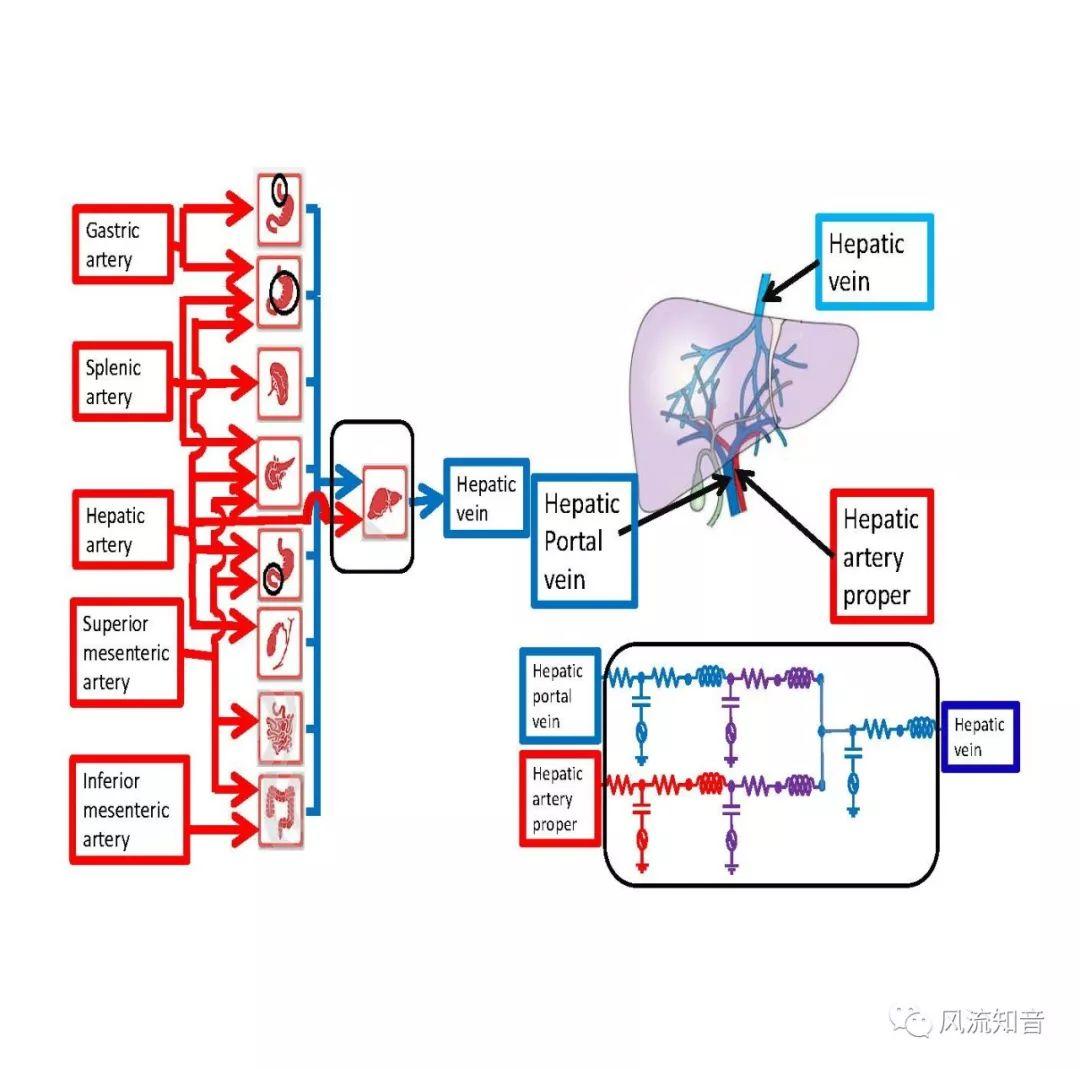
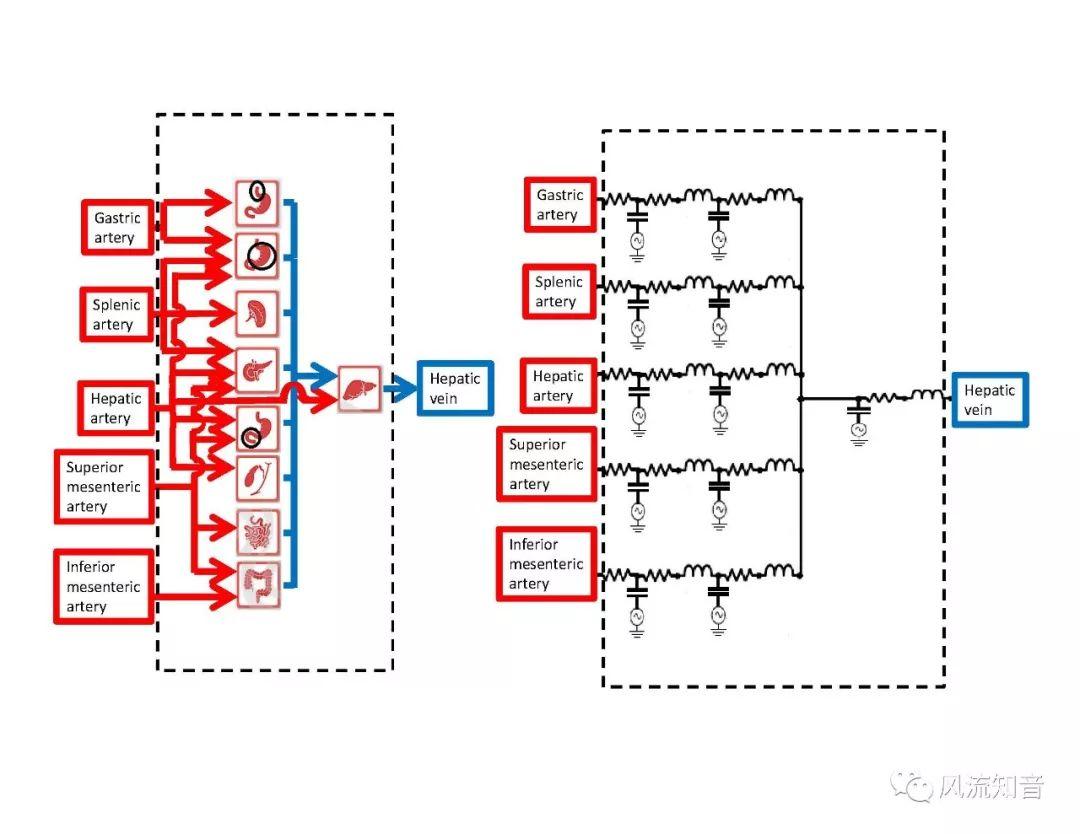
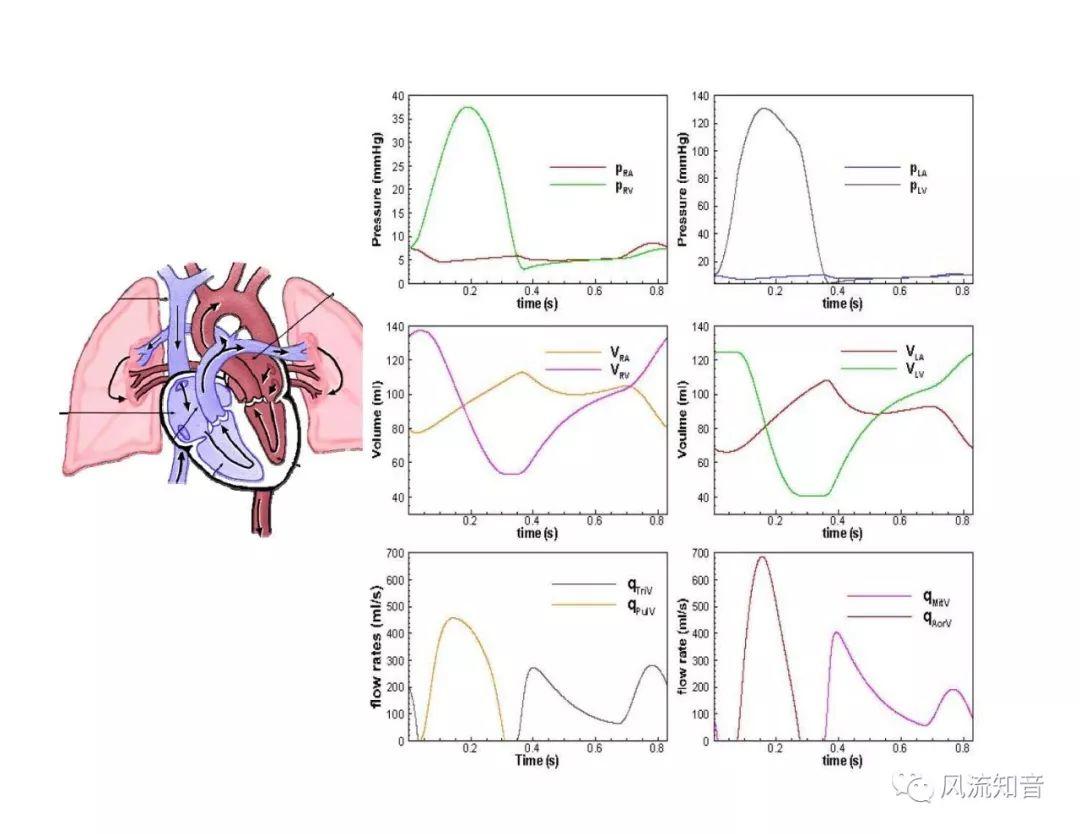
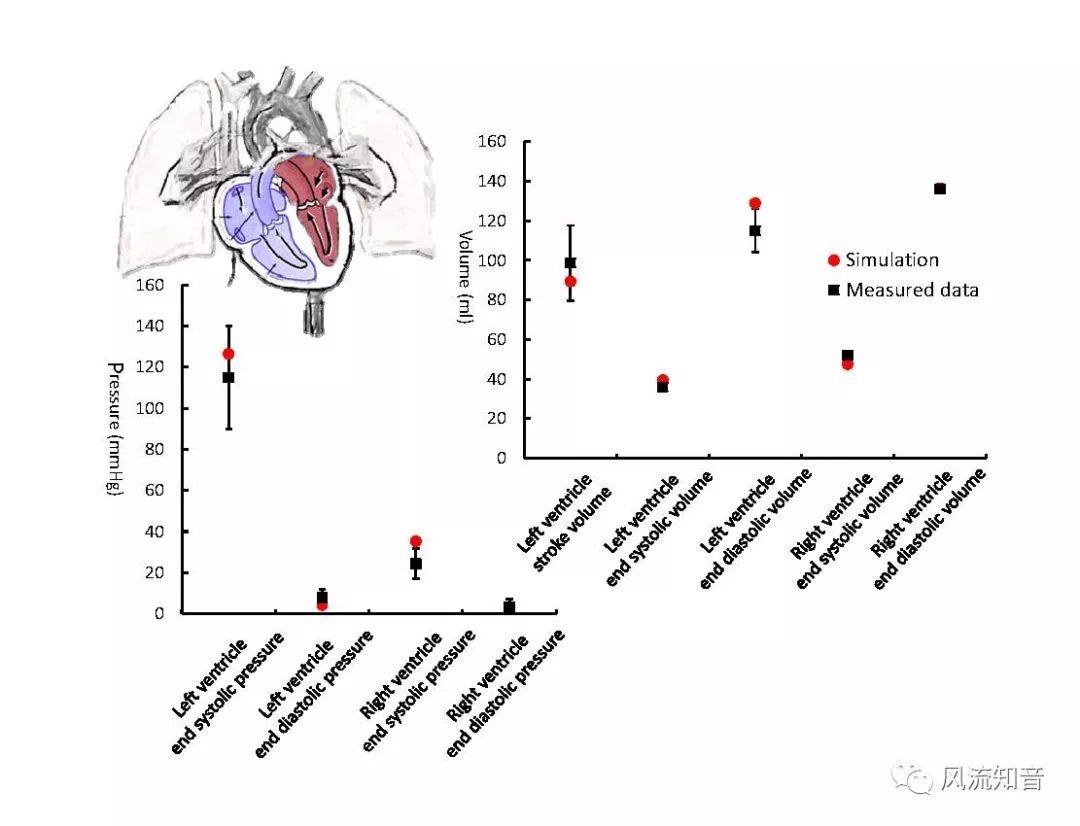
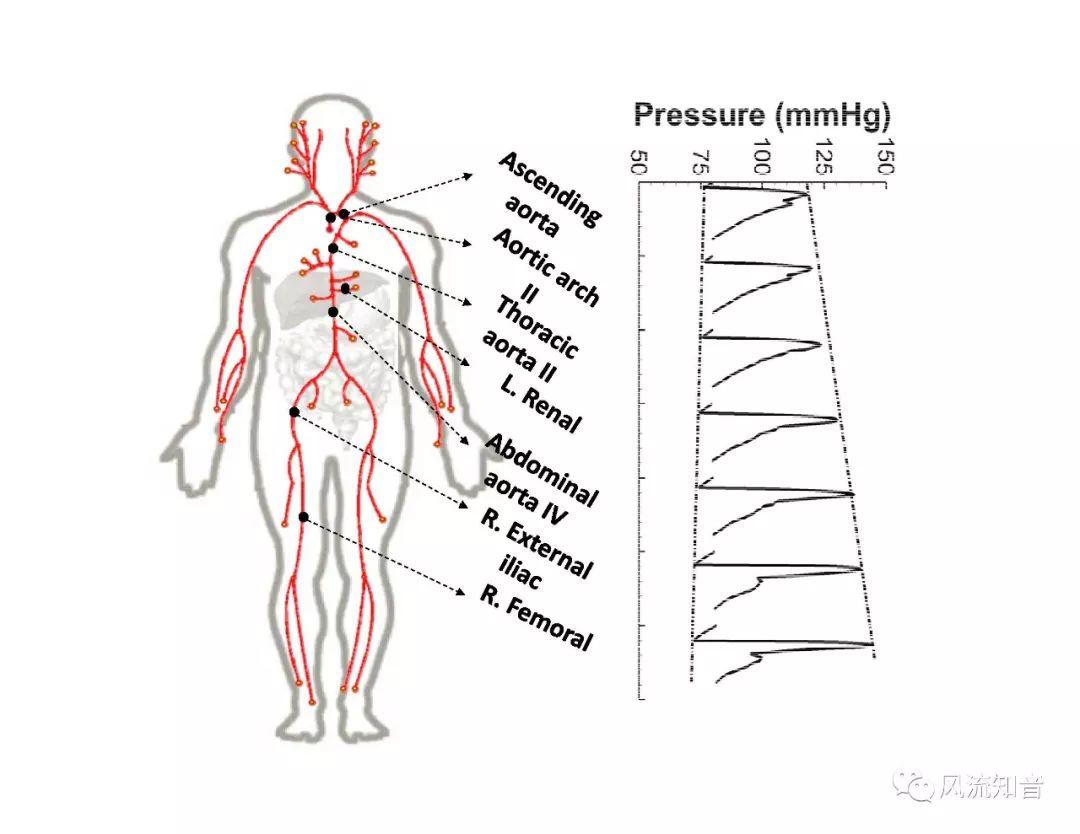
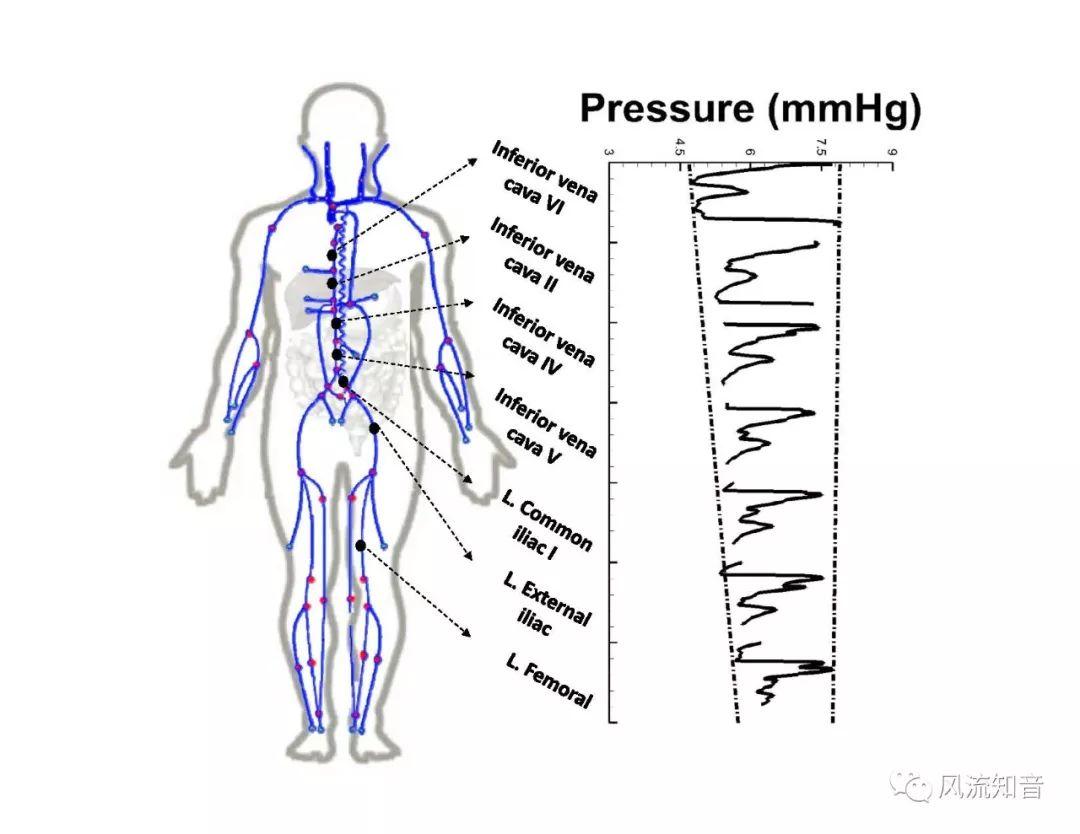
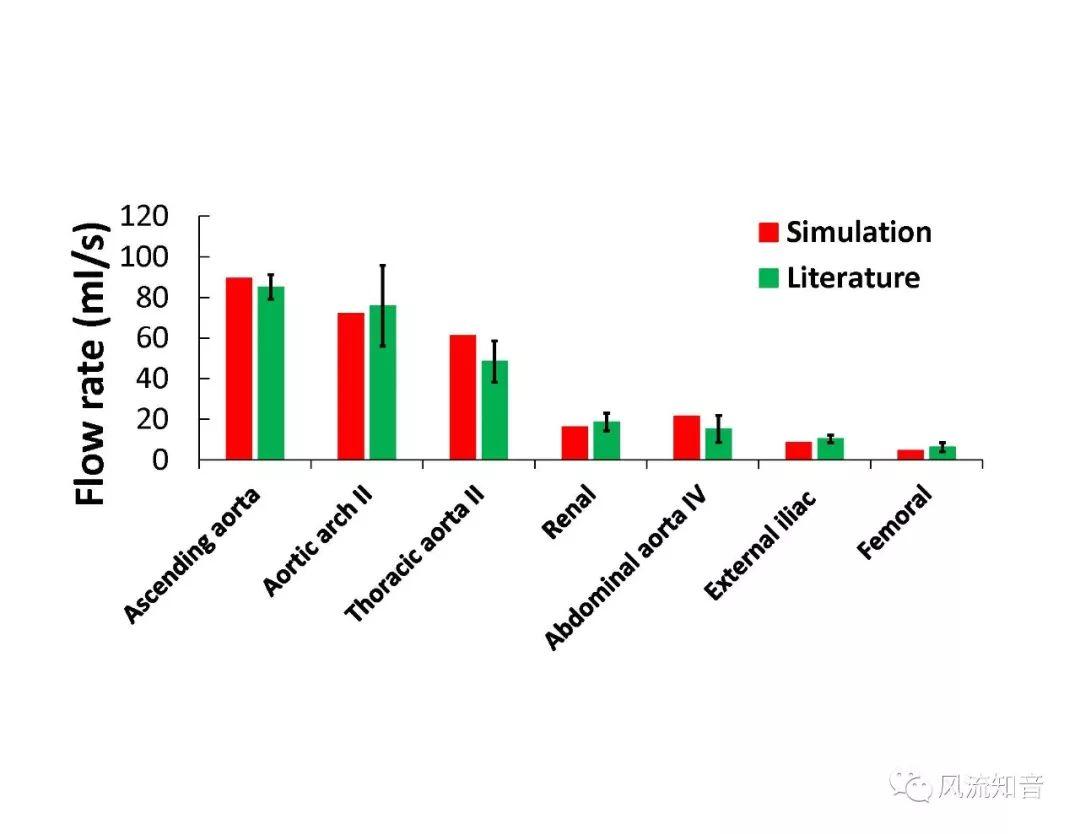
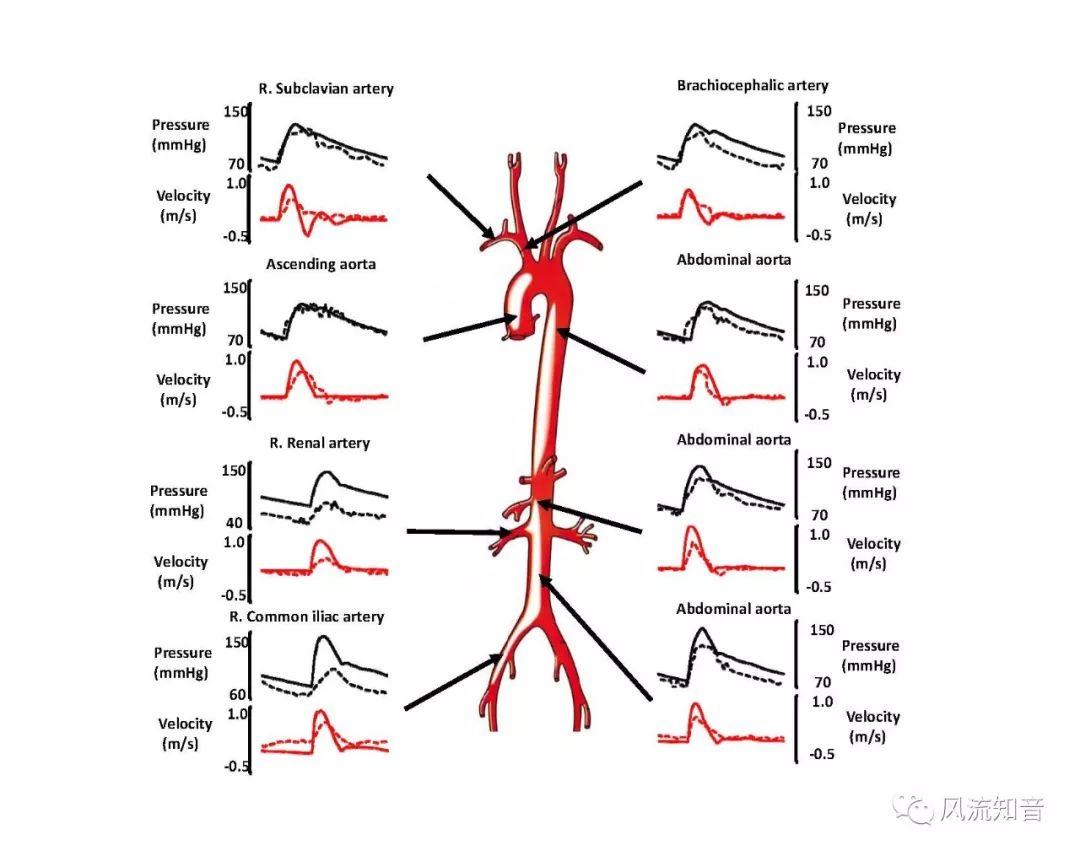
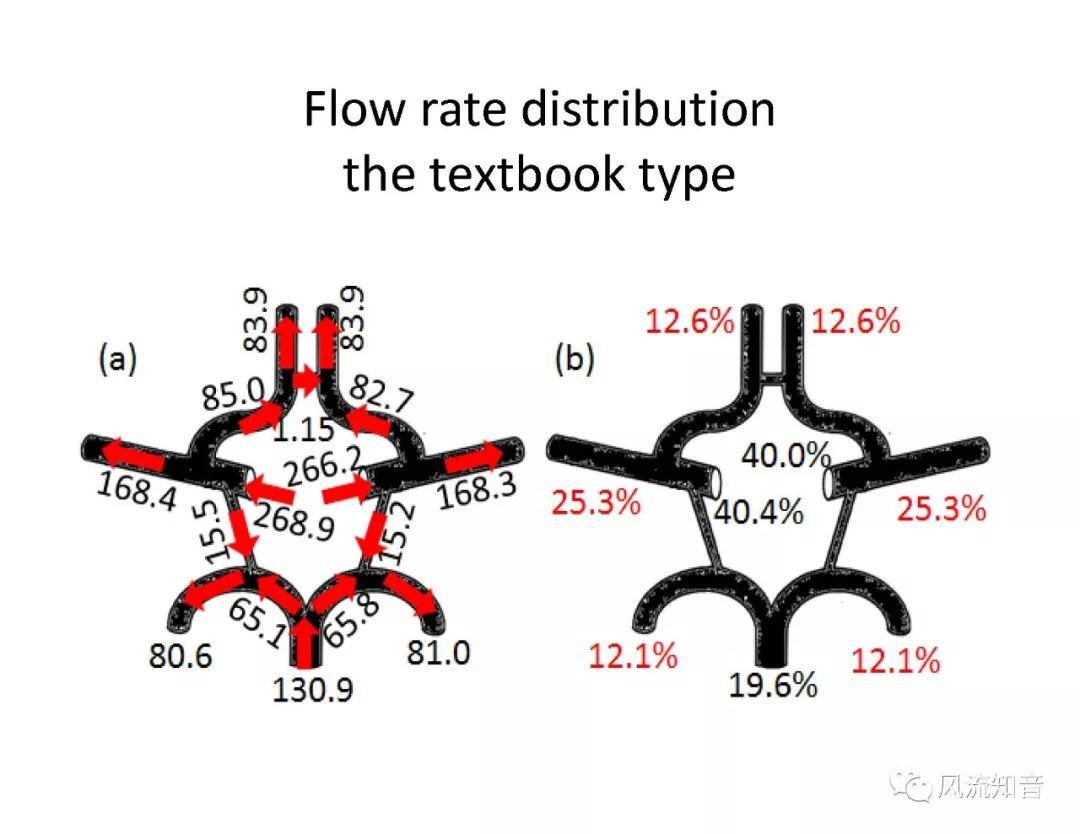
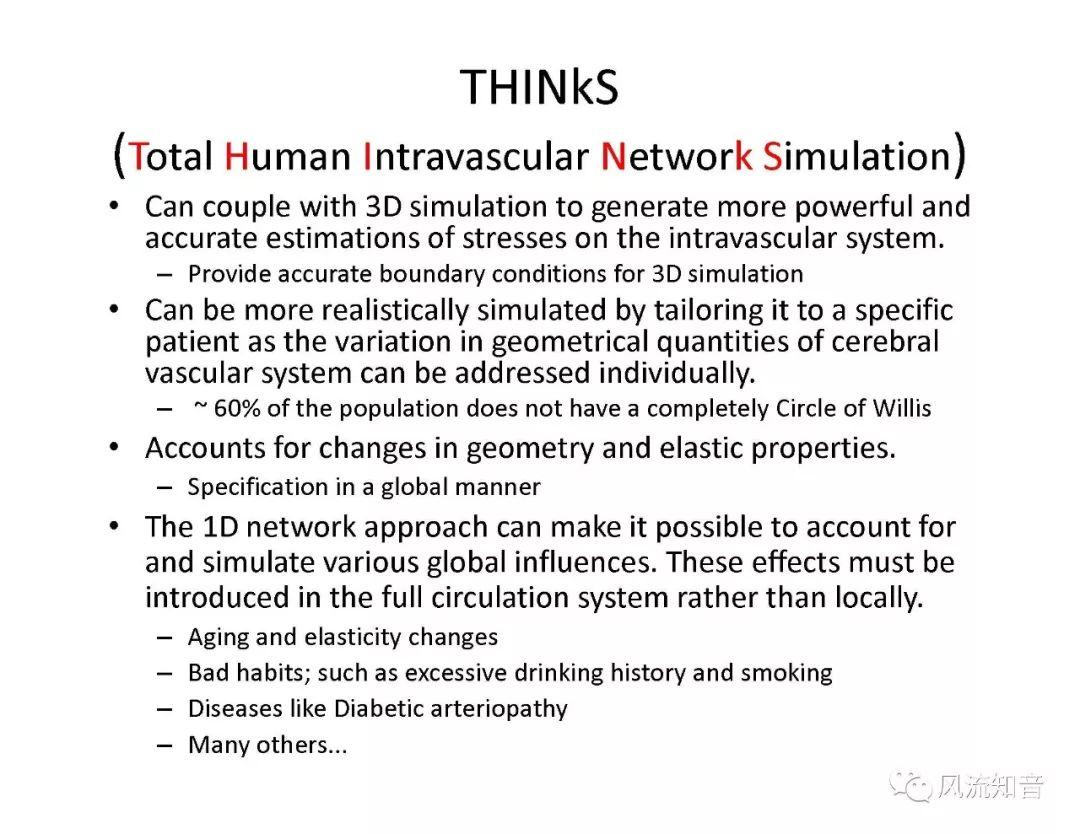
A 1D simulation of the complete vessel network, THINkS (Total Human Intravascular Network Simulation), is introduced to improve the accuracy of computational fluid dynamic (CFD) calculations and better define boundary conditions of the complex human vessel network. THINkS is a 1D network simulation of the complete human circulatory system. It consists of a simulation of 85 major arteries, 158 major veins, 43 arterial and 77 venous junctions. Blood flow in arterioles, capillaries and venules is modeled using lumped parameter models, or the 0D models, which are modeled using the connection of a number of capacitors, resistors and inductors to represent the real physics. The model used a simple 0D model for 20 one-artery-to-one-vein micro circulations and 4 other 0D models for 7 complex arteries-to-veins micro circulations. Moreover, a 4-chamber 0D model for the heart is used to allow blood to pump from superior vena cava I and inferior vena cava I veins, through the pulmonary system, and discharge back to the ascending aorta artery. In addition to the 4 valves inside the heart, there are also 15 venous valves used in the venous system. THINkS calculates the complete human blood network circulation and it indicated usefulness of the model in predicting the trends of cerebral flow patterns.
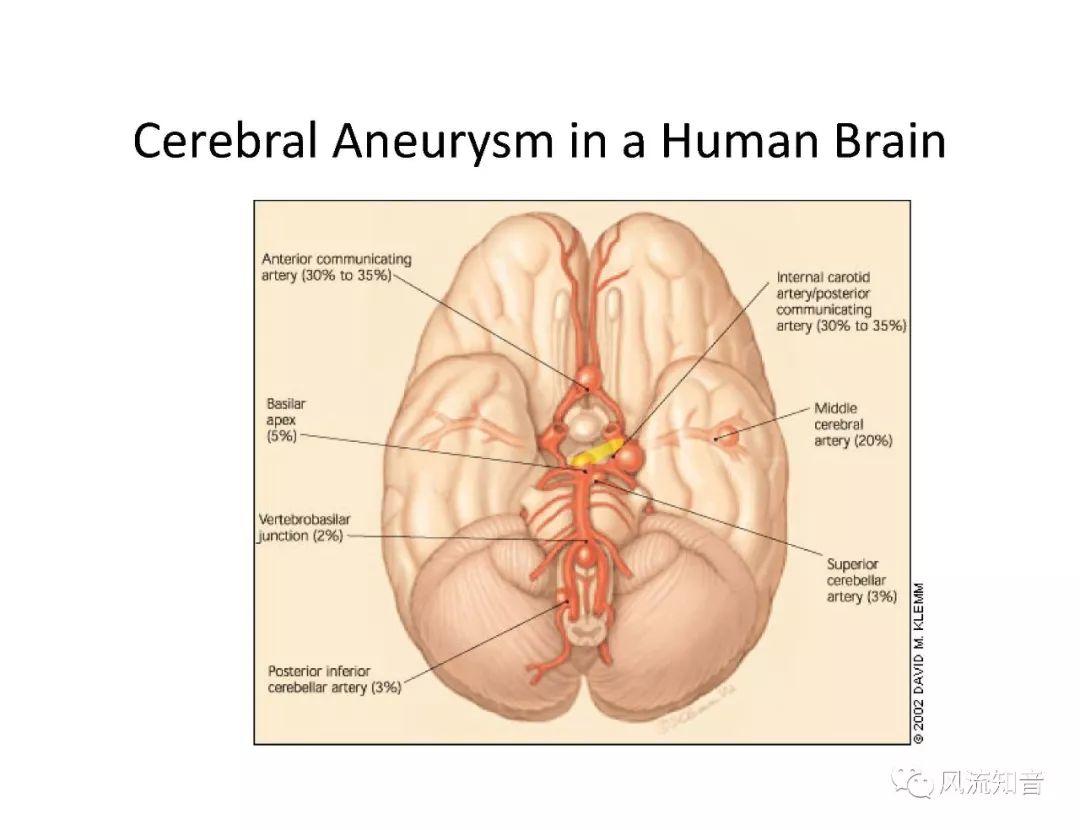
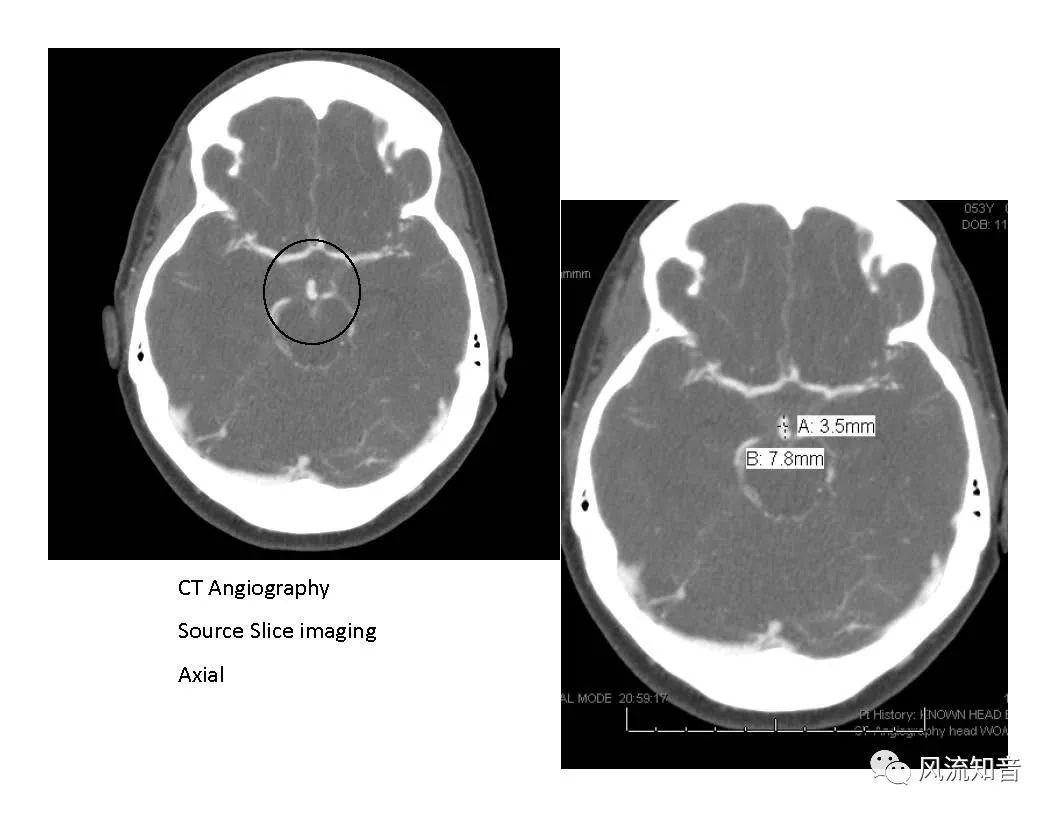
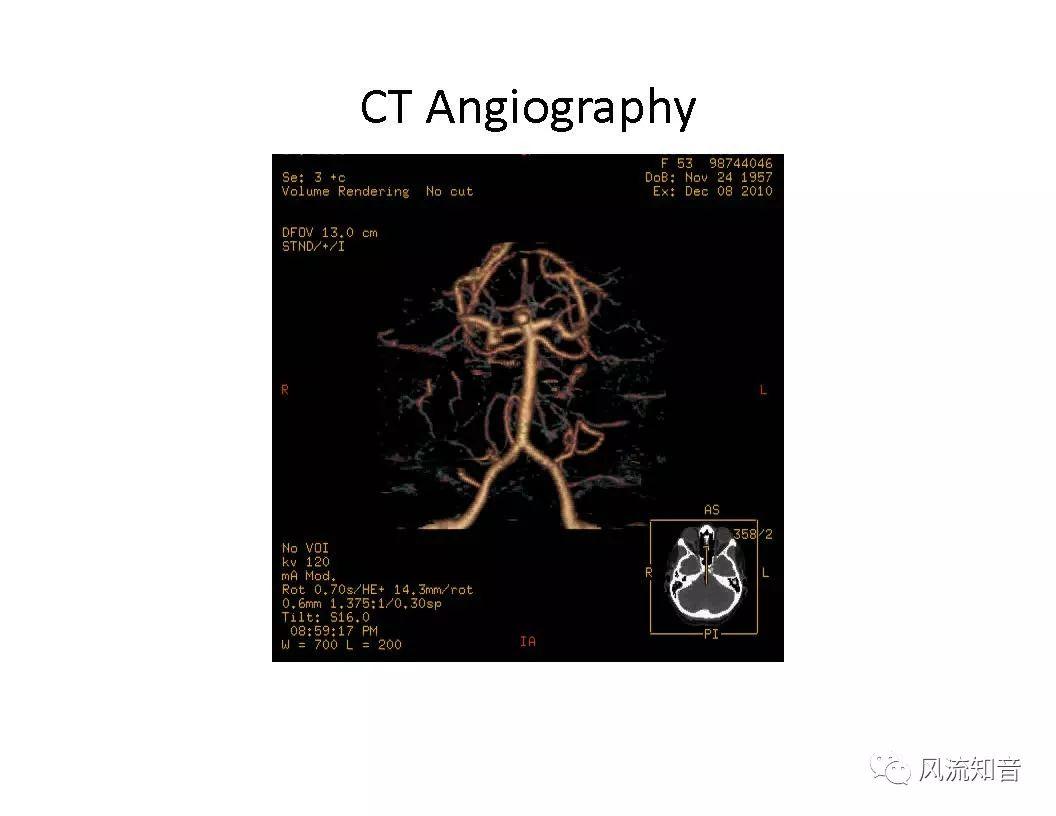
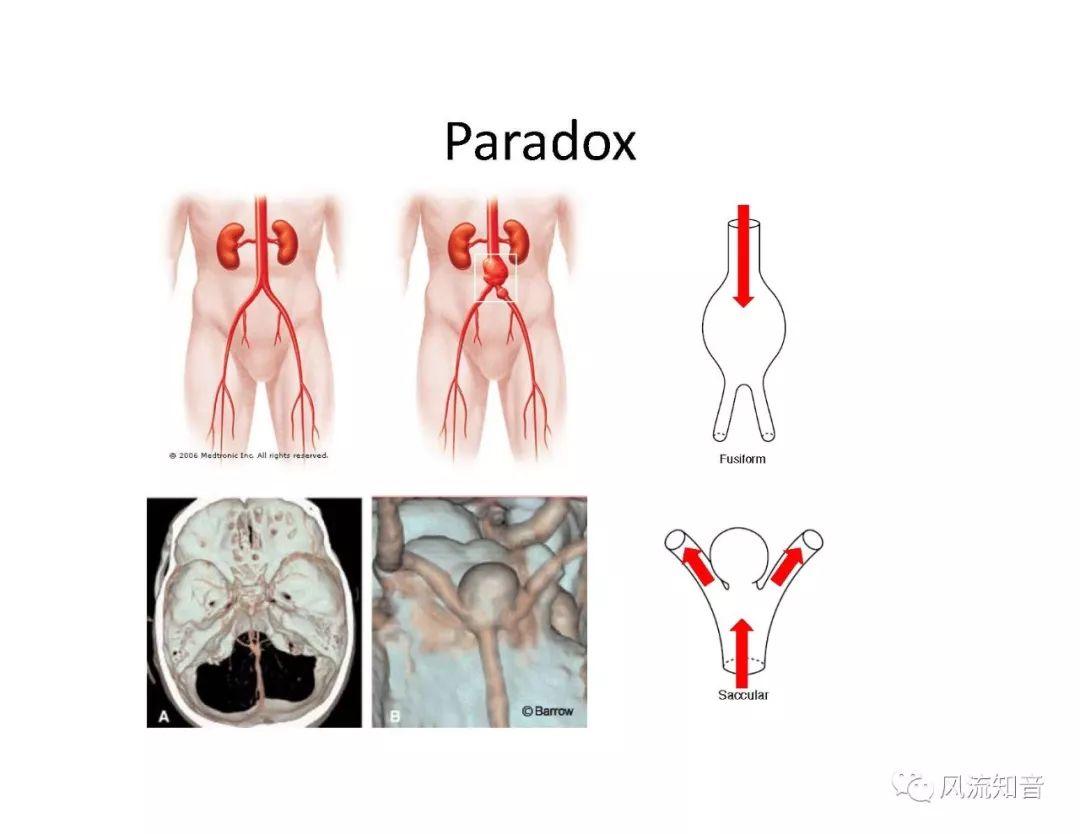
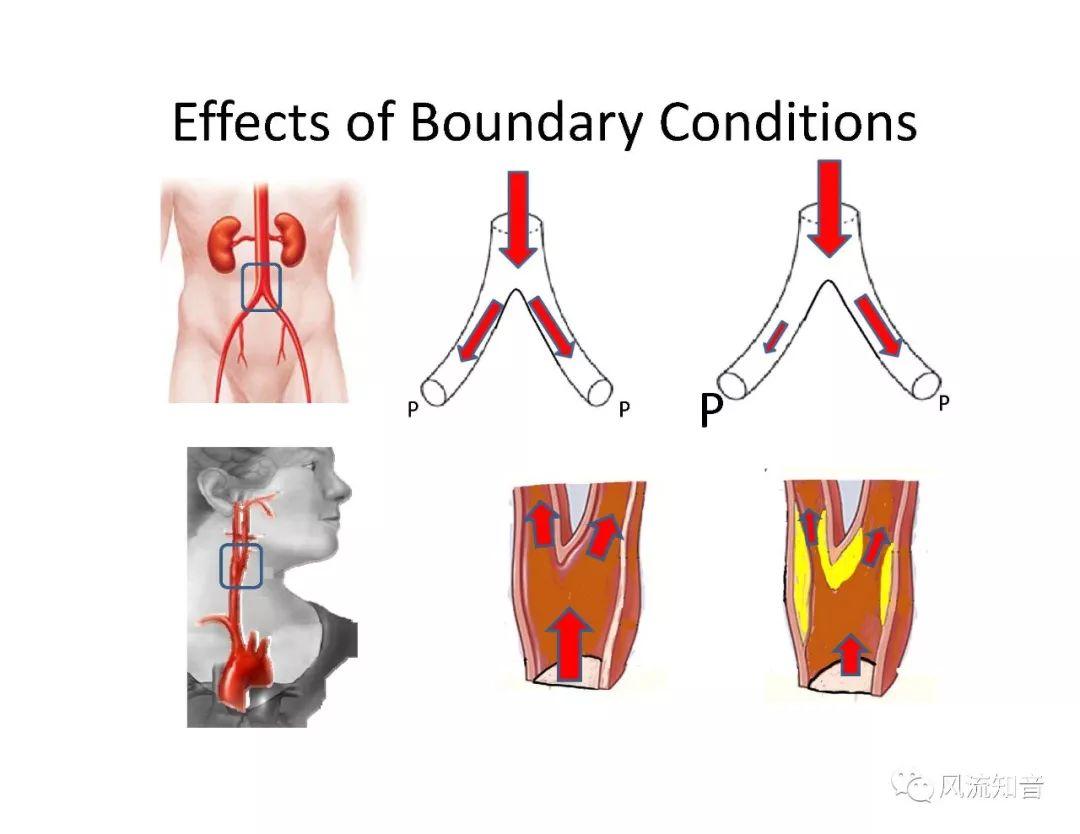
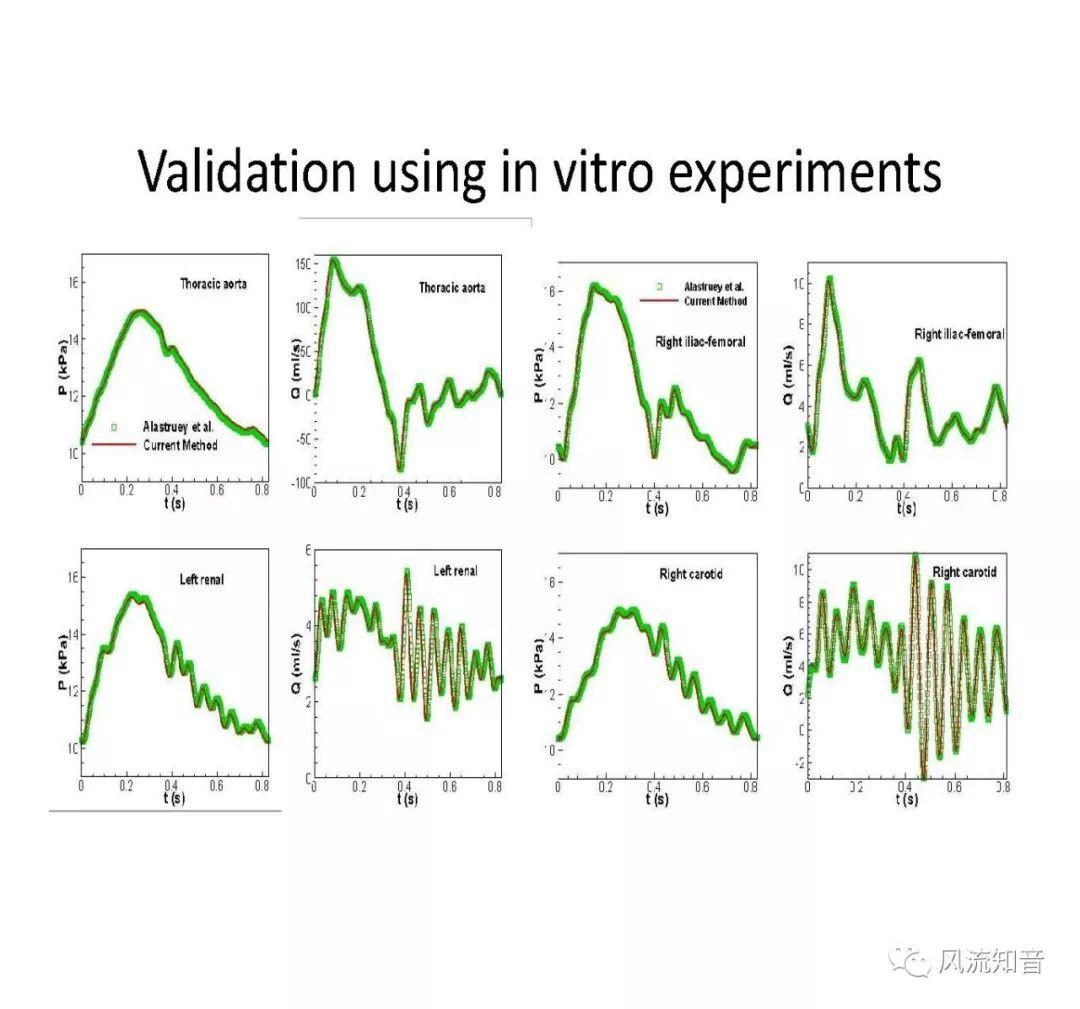
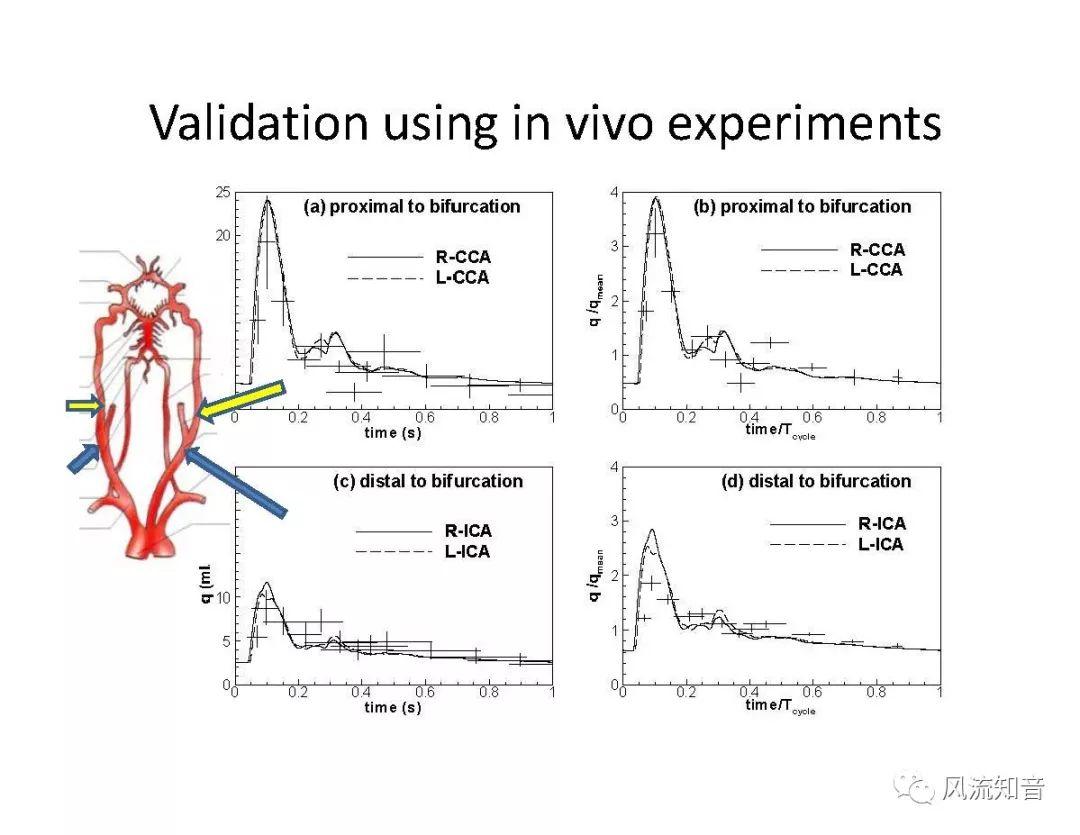
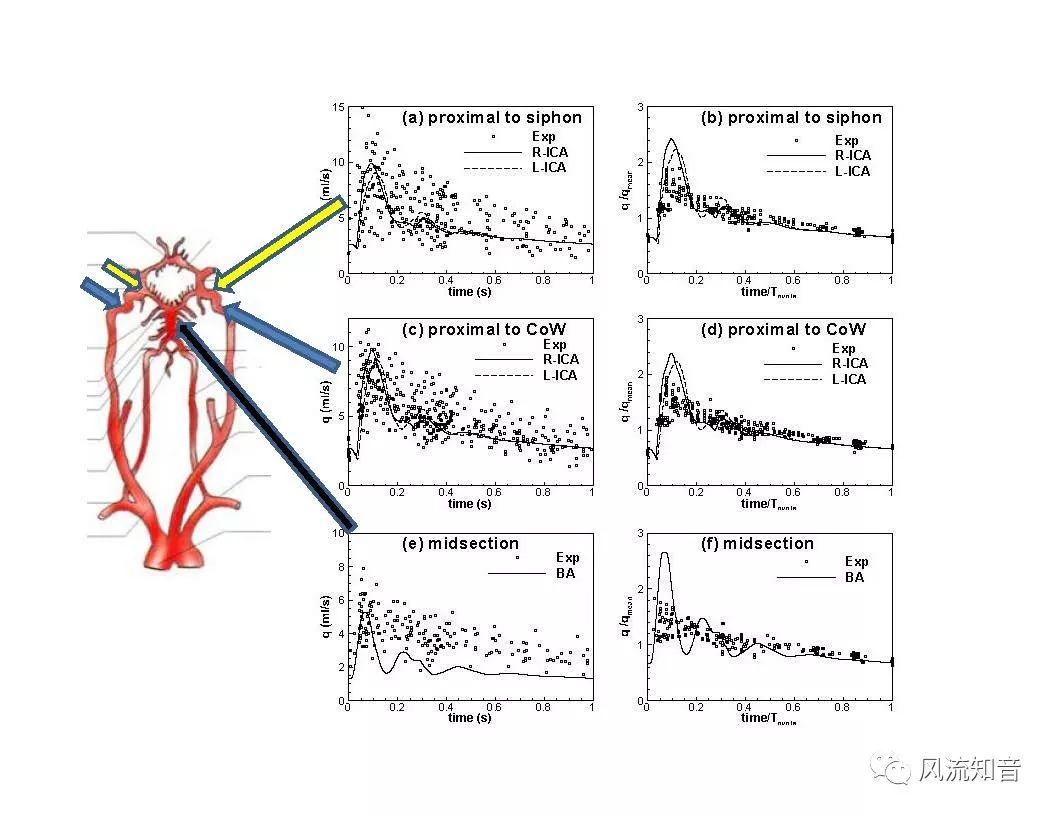
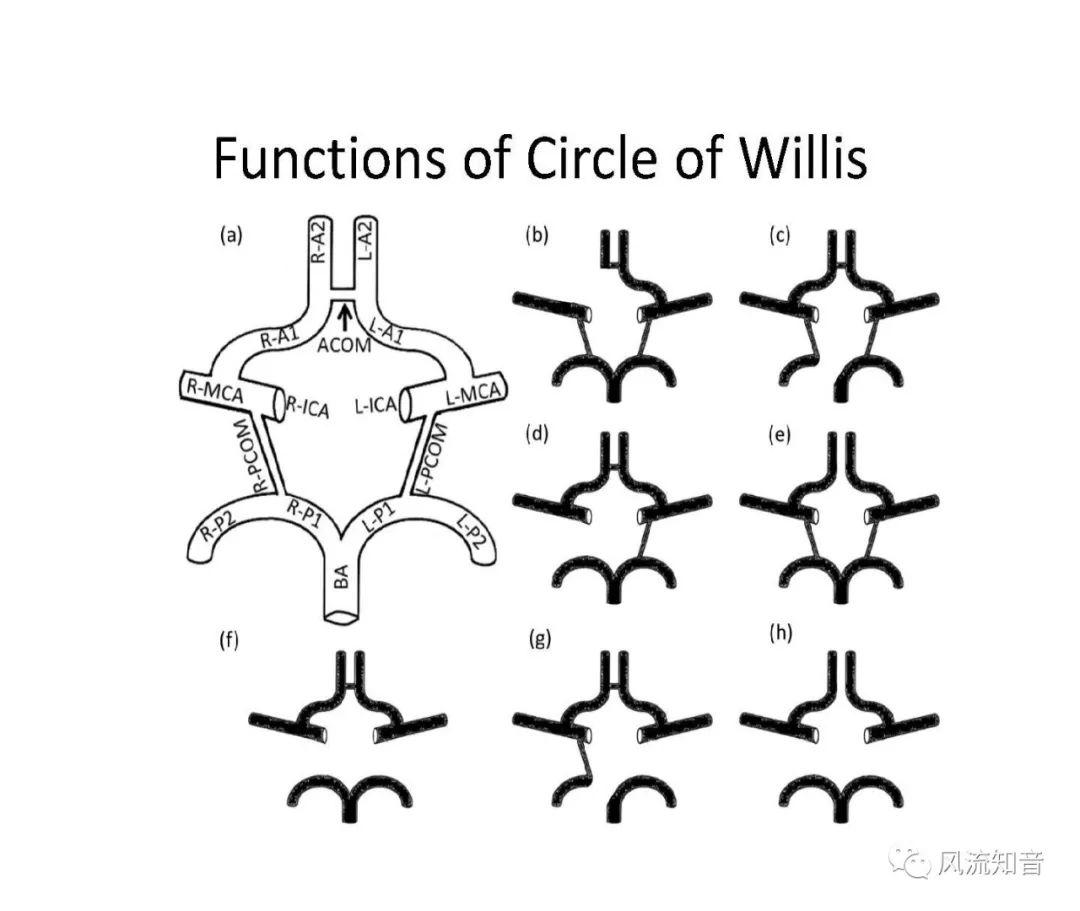
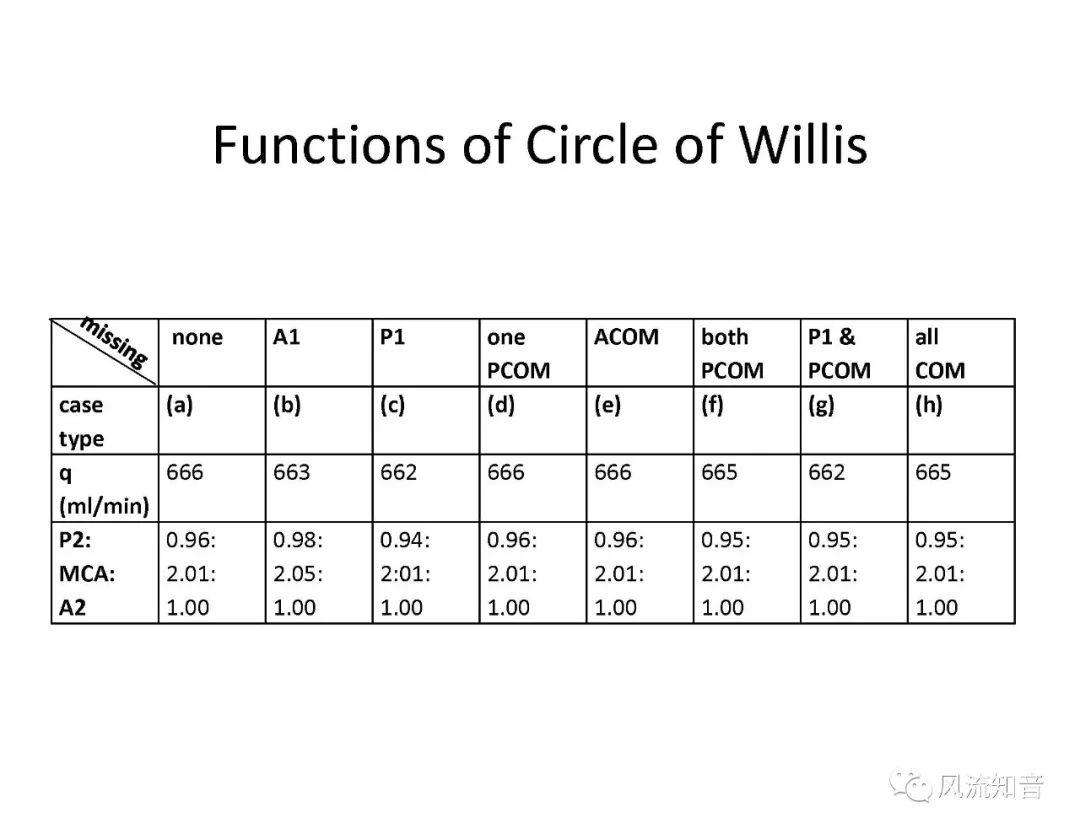
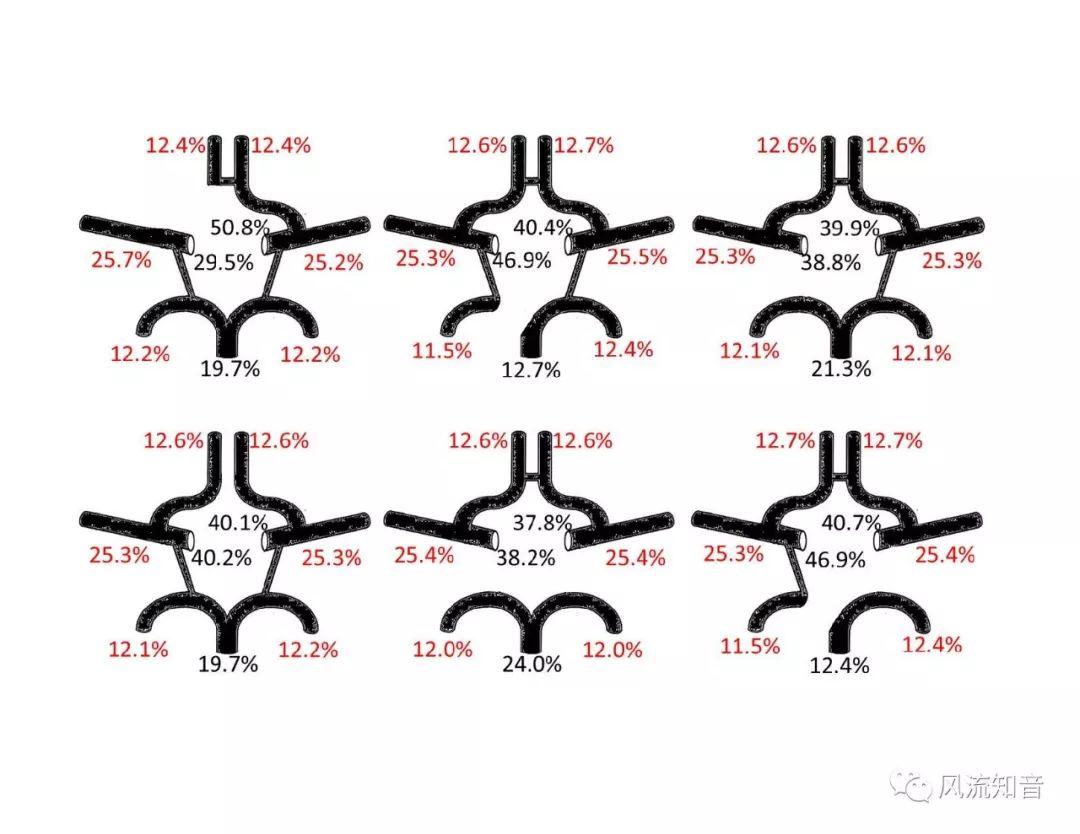
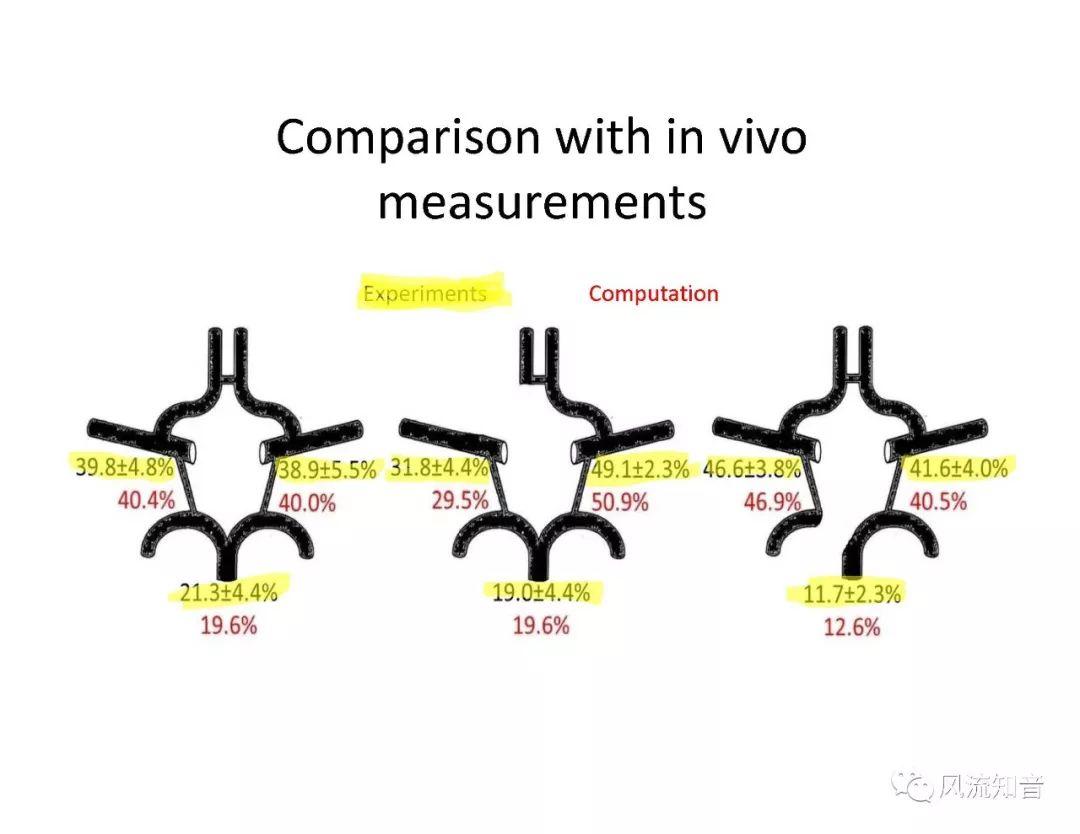
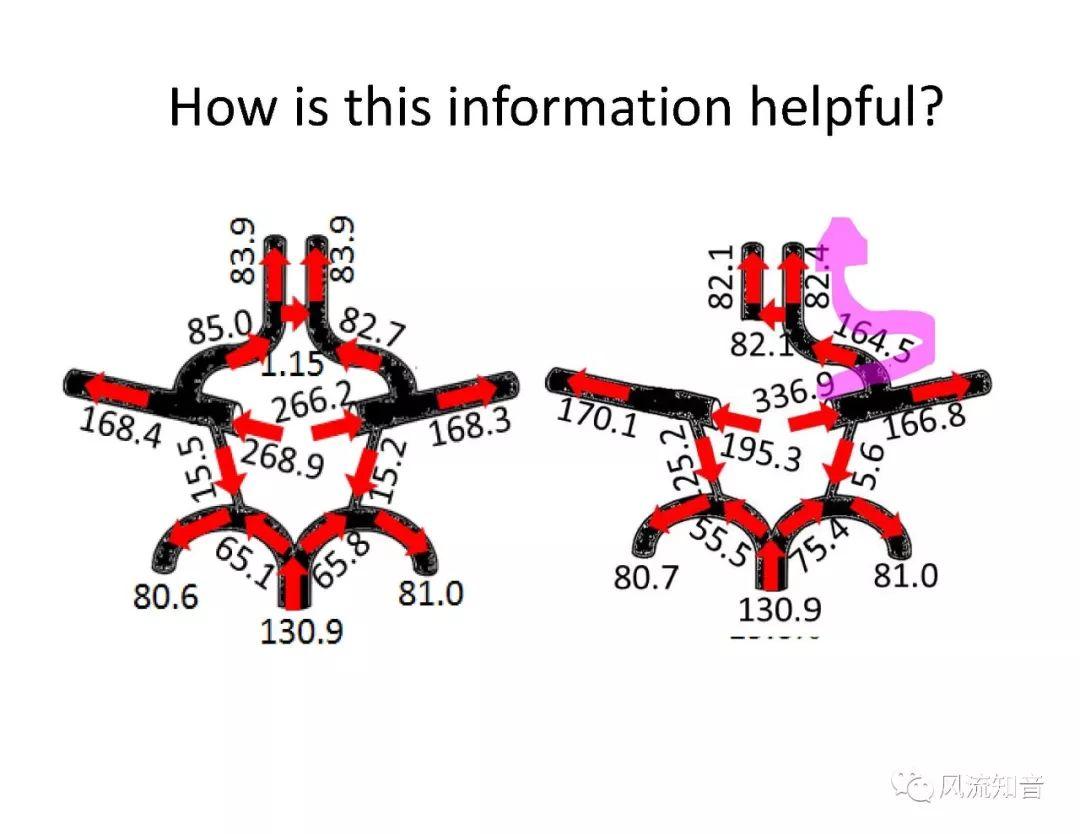
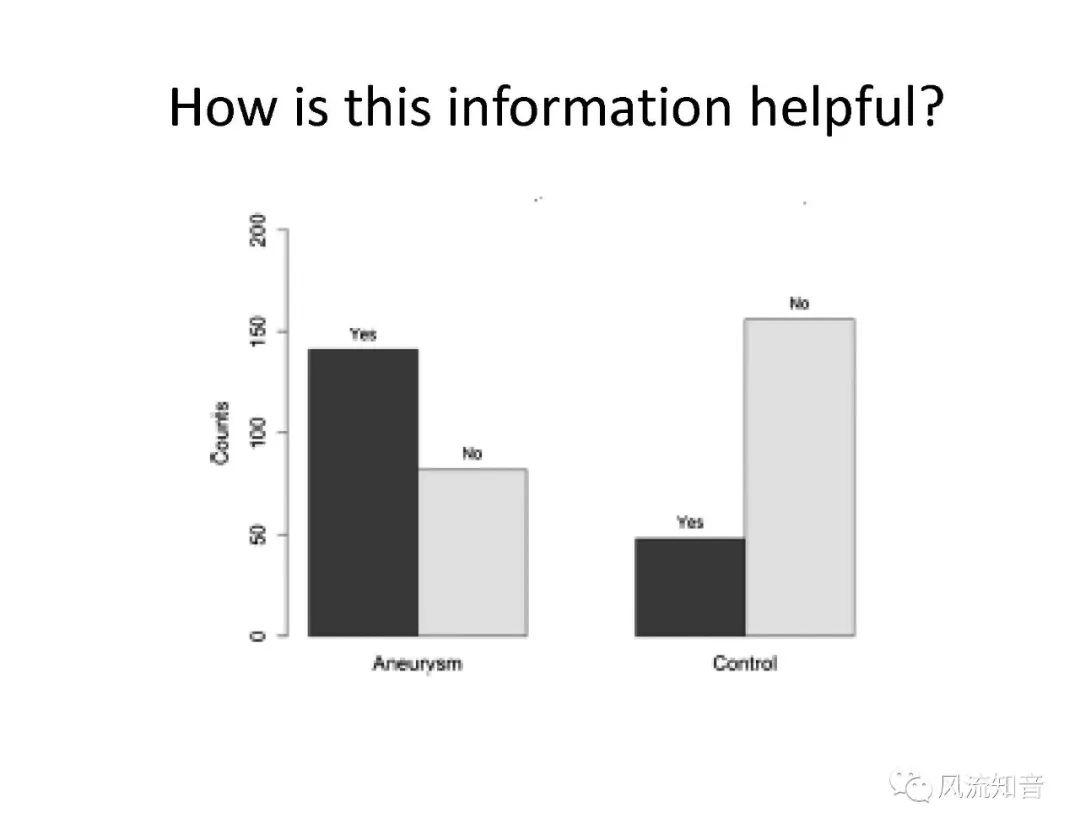
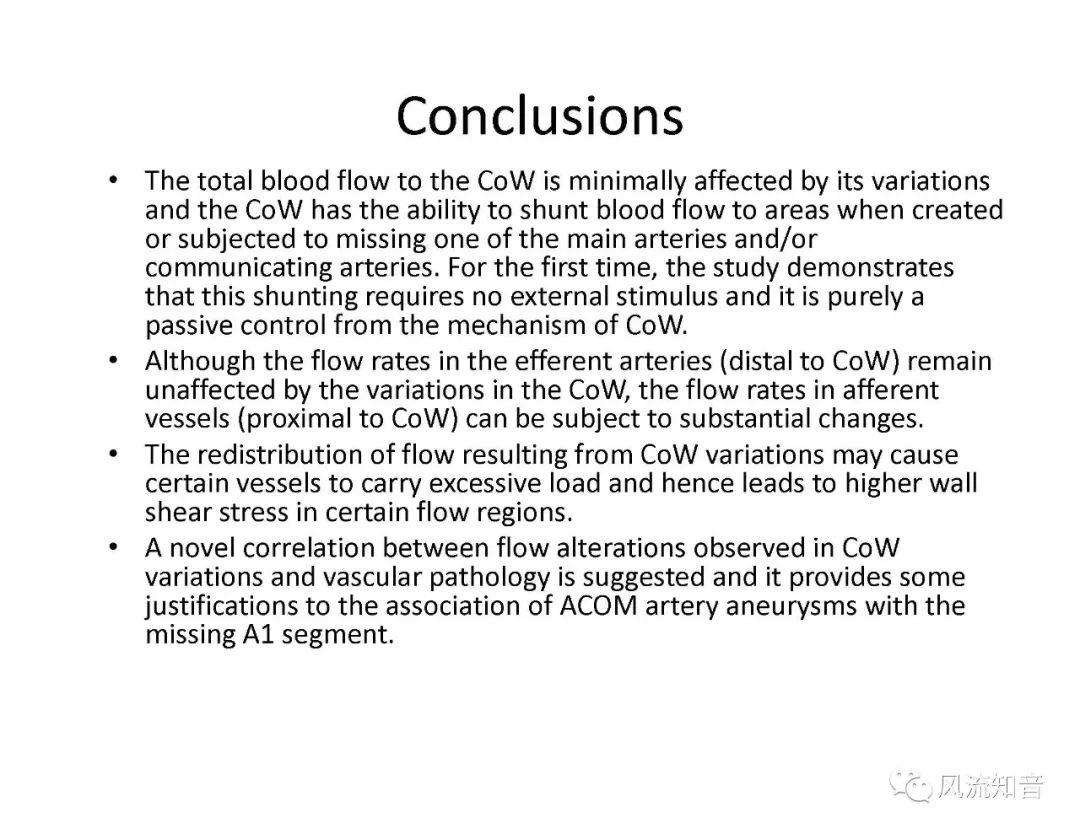
THINkS has been validated against experimental data and shown to respond correctly to the flow pattern changes caused by variations in the Circle of Willis (CoW). The overall trends of the simulation results for estimating flow rates in the missing A1 and the missing P1 CoW variations are confirmed by the in vivo experimental data.A test of a wide range of CoW variations has suggested that the ring-like vessel connection can regulate the blood flow to enable different parts of brain to receive the same blood flow rates. The mechanics are built into the ring-like structure (passive regulation) and require no external stimulus (active regulation)?. Although the flow rates in efferent arteries remain unaffected by the variation of CoW, the flow rates in afferent vessels can subject to substantial changes. The redistribution of flow due to CoW variation may cause some vessels to carry excessive load, leading to high wall shear stress in certain flow regions. This provides an explanation why the CoW variation (missing A1) is correlated with a higher prevalence of anterior communicating artery aneurysms. A new direction of research to correlate CoW variations to different vascular diseases by taking into consideration the redistribution of the flow is therefore suggested.